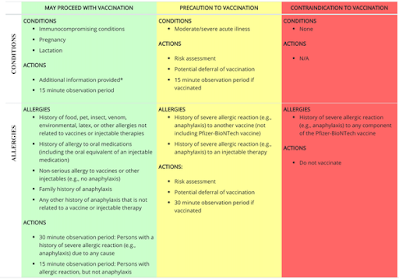
precaution in vaccination
If you have actually a background of hatreds food, family pet dogs, bugs or various other points, the Facilities for Illness Manage and Avoidance suggests that you wage inoculation, with an monitoring duration. If you have actually a background of serious allergy, or what is called anaphylaxis, to one more injection or injectable treatment, your physician could do a danger evaluation, defer your inoculation, or continue and after that observe you after inoculation. The just need to prevent inoculation is a serious allergy to any type of element of the COVID-19 injection. The CDC has particular suggestions for post-vaccine monitoring.
The CDC and Food and Medication Management motivate the general public to record feasible unfavorable occasions to the Injection Unfavorable Occasion Coverage System, or VAERS. This nationwide system gathers these information to appearance for unfavorable occasions that are unforeseen, show up to occur more frequently compared to anticipated or have uncommon patterns of incident. Anybody that has skilled an unfavorable occasion ought to record it to the system.
Coverage an unfavorable occasion is an essential action to guaranteeing security and to assist the CDC check the vaccines. Security is a leading concern, and researchers and public health and wellness authorities have to learn about unfavorable responses.
An unfavorable occasion is various in many situations from a common injection adverse effects. Vaccines might trigger a adverse effects, such as discomfort at the shot website or inflammation. Unfavorable occasions are much a lot extra major and could in some cases be deadly. If you're uncertain whether you have skilled a adverse effects or unfavorable occasion, you could still record the occasion.
Individuals are provided a truth sheet when they are vaccinated. Healthcare service companies that vaccinate individuals will be needed to record to VAERS specific unfavorable occasions complying with inoculation. Additionally, under the regards to the emergency situation utilize permission, healthcare service companies likewise should comply with any type of modified security coverage demands that might occur.
The CDC is likewise executing a brand-new smartphone-based device called v-safe to sign in on people's health and wellness after they get a COVID-19 injection. When you get your injection, you ought to likewise get an info sheet informing you ways to register in v-safe. If you register, you'll get routine text guiding you to studies where you could record any type of issues or unfavorable responses you have after getting a COVID-19 injection. Tips Terbaik Menang Bermain Judi Bola Online
It's most likely to be a number of months. The presently licensed Pfizer and soon-to-be-authorized Moderna injection are not appropriate for kids. Much a lot extra research study and medical tests have to be done to consist of more youthful kids in COVID-19 injection tests.
Inning accordance with the American Academy of Pediatric medicines, Pfizer has registered kids to age 12 and sent a ask for emergency situation utilize permission for inoculation to age 16. Moderna, whose injection is anticipated to get emergency situation utilize permission from the FDA any type of day, will begin a comparable examine.
In the Unified Kingdom, AstraZeneca has authorization to register kids ages 5 to 12 in medical tests, however the pharmaceutical business has not yet registered any type of kids in tests in the U.S.